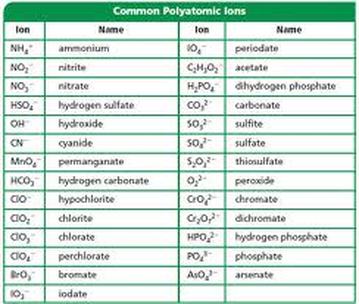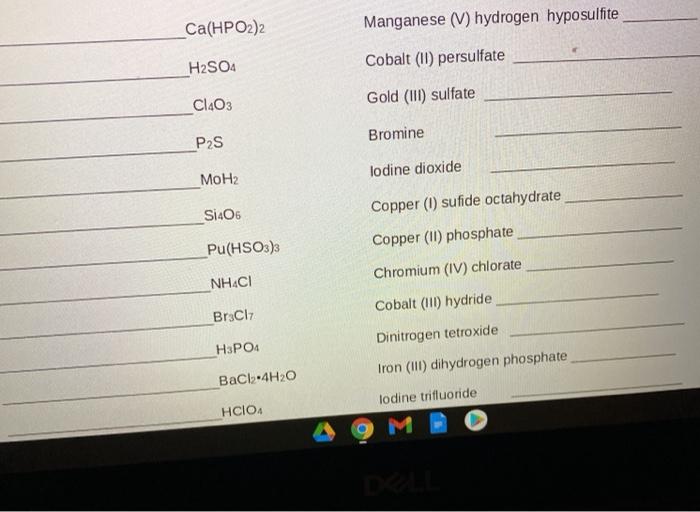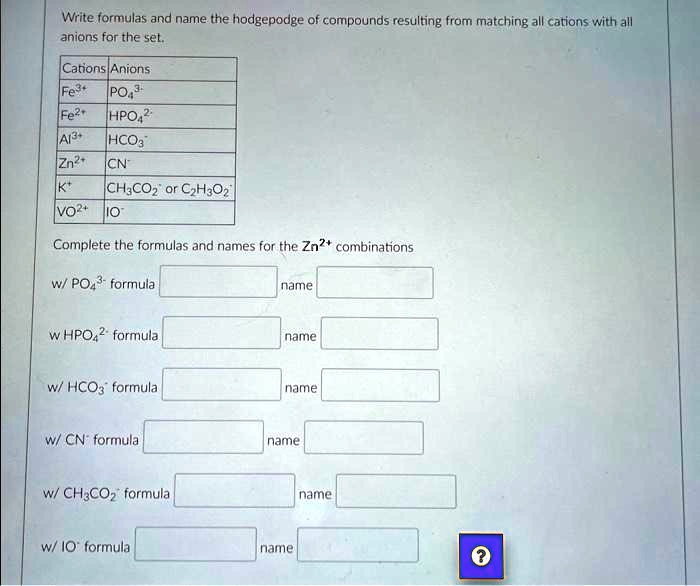
SOLVED: Texts: Write formulas and name the hodgepodge of compounds resulting from matching all cations with all anions for these set. Cations: Fe3+, PO3-, Fe2+, HPO42-, Al3+, HCO3-, Zn2+, CN-, K+, CH3COO-,
Draw the Lewis dot structures of (PO4)3 ,(HPO3)2 and (H2PO2) and mention Co ordinate bonds wherever required.
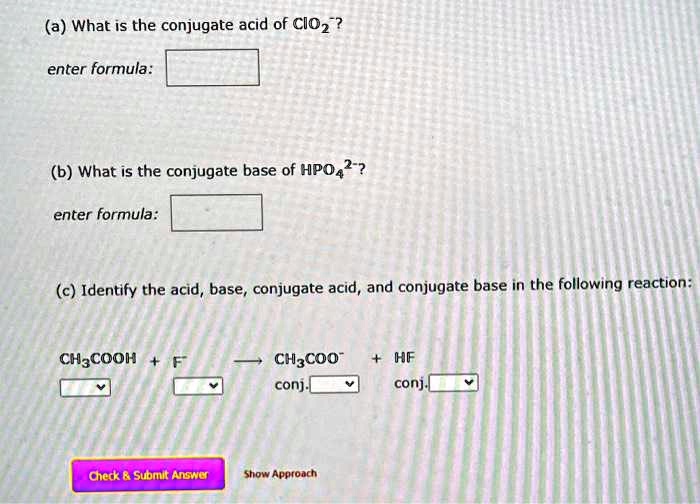
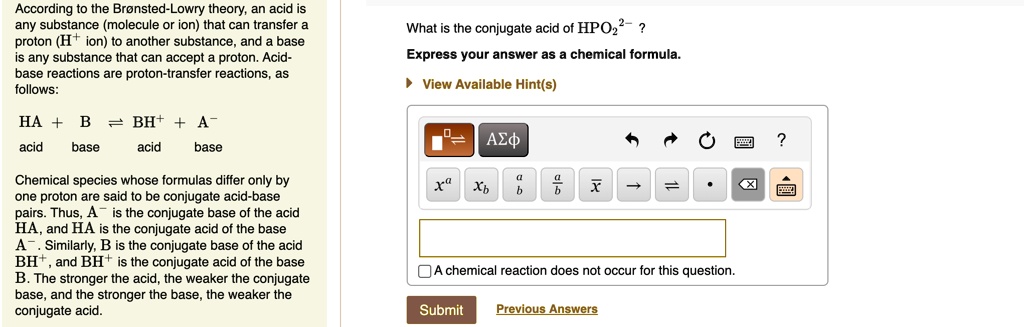
SOLVED: (a) What is the conjugate acid of ClO-? Enter formula: (b) What is the conjugate base of HPO2-? Enter formula: (c) Identify the acid, base, conjugate acid, and conjugate base in
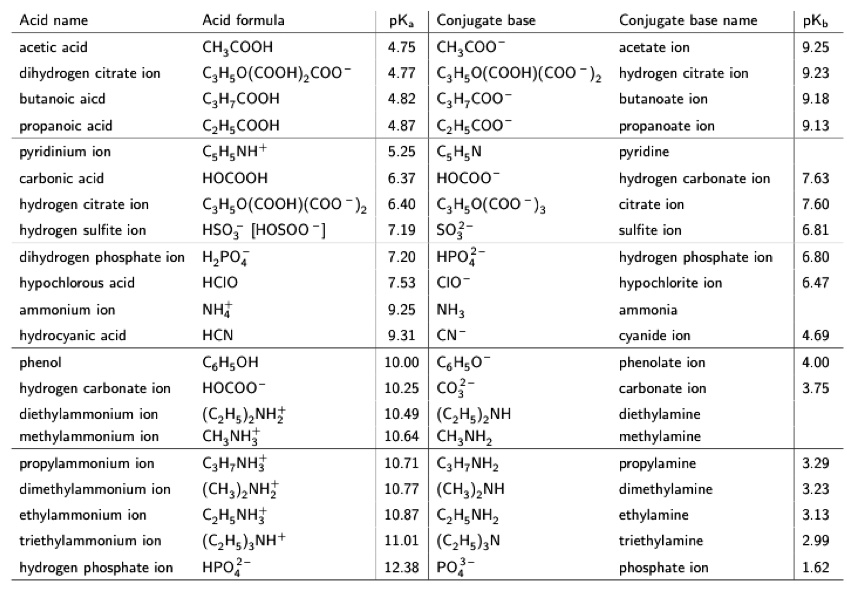
SOLVED: Text: Acid name Acid formula acetic acid CH3COOH dihydrogen citrate ion C6H8O7(COOH) COO- butanoic acid C4H8COOH propanoic acid C3H6COOH pyridinium ion C5H5NH+ carbonic acid H2CO3 hydrogen citrate ion C6H6O7(COOH)(COO-) hydrogen sulfite