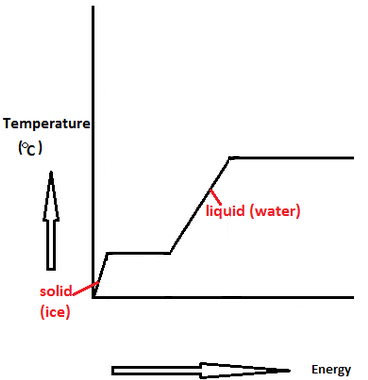
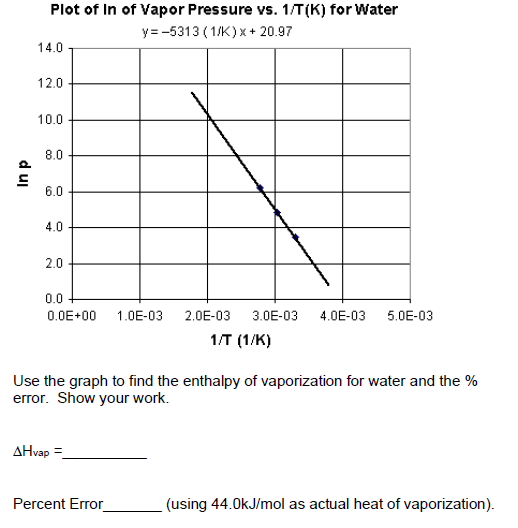
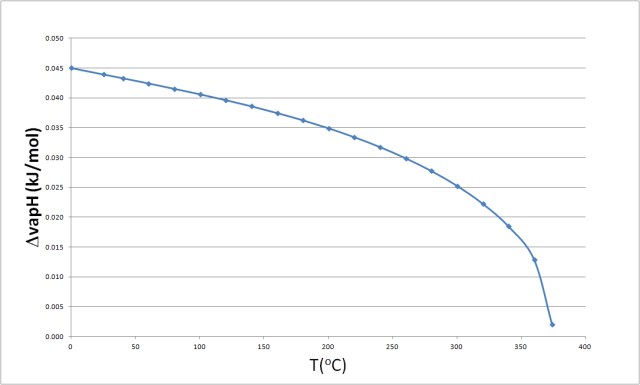
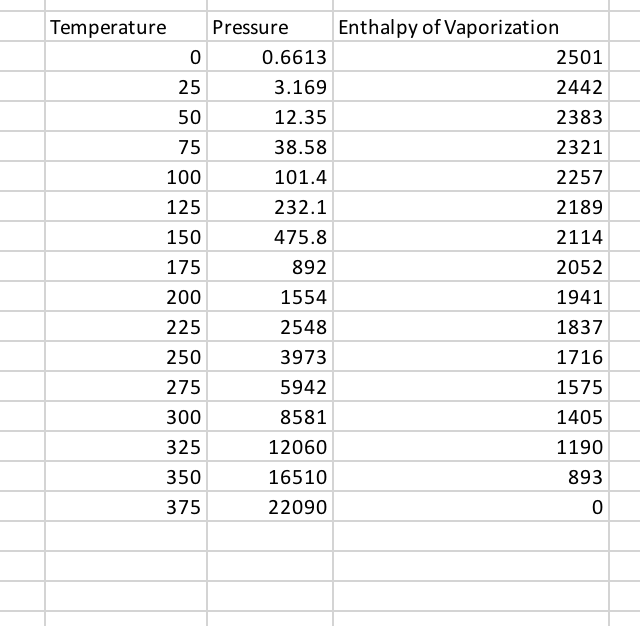
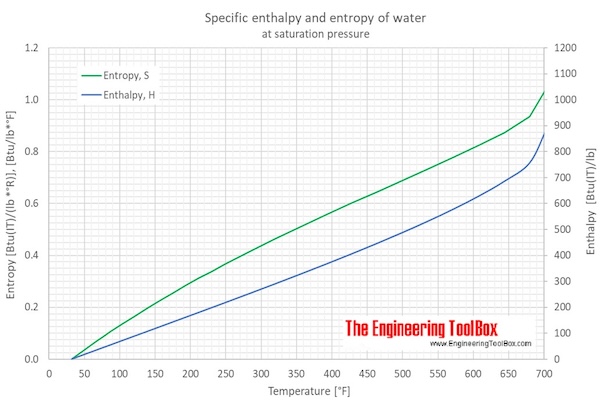
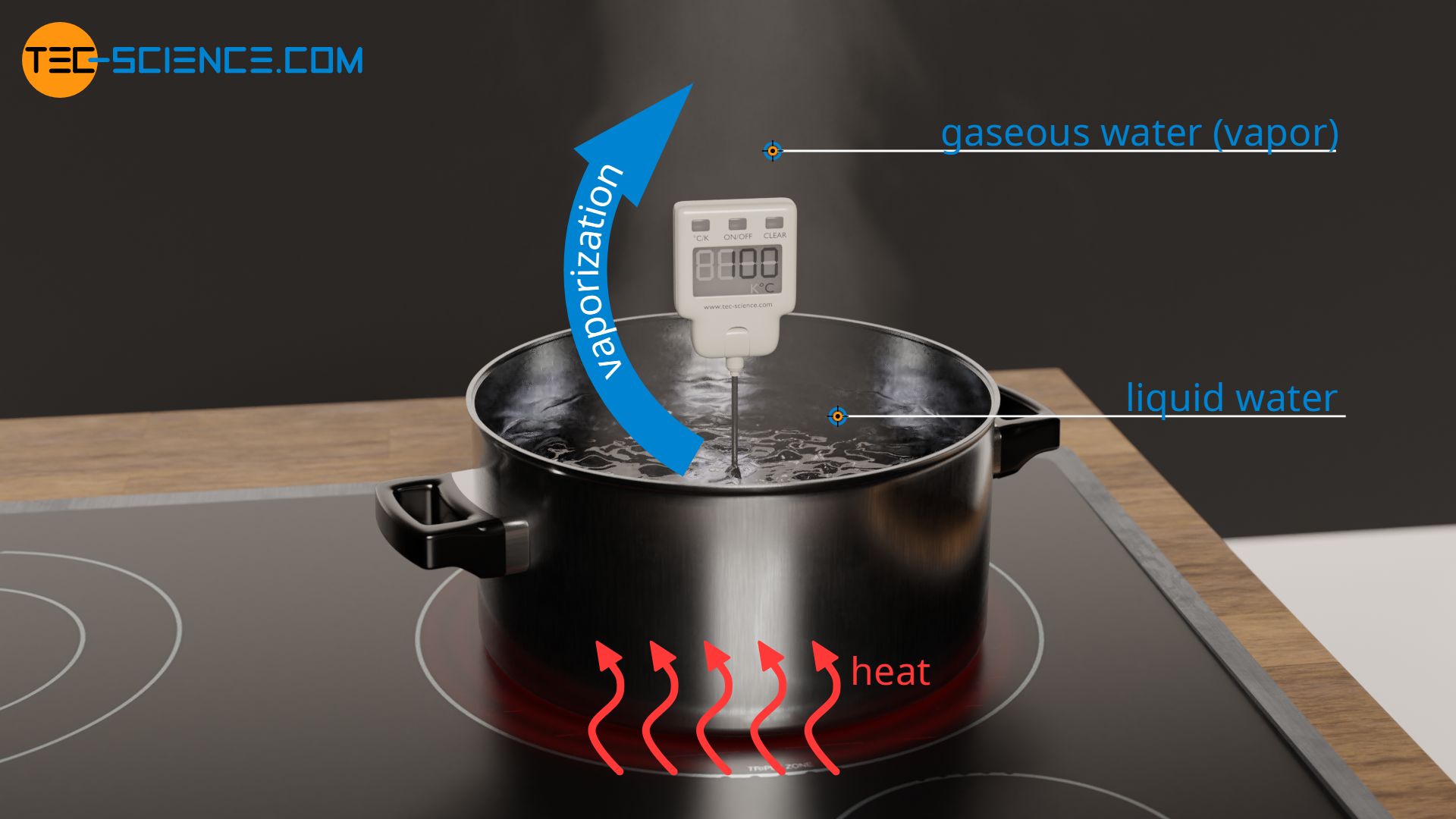
SOLVED: Find the enthalpy of vaporization of water in kJ/mole from vapor pressures determined at the following two temperatures: At 40.0 o C, vapor pressure = 55.3 torr; at 80.0 o C,

Enthalpy of vaporization of water: (—) Reference fundamental equation... | Download Scientific Diagram
Calculate the molal elevation constant of water if molar enthalpy of vaporisation of water at 373 K is 40.585 kJ/mol.
Molar enthalpy of vaporization of water from triple to critical points. | Download Scientific Diagram
Standard enthalpy of vaporisation ΔvapH° for water at 100°C is 40.66 kJ mol^-1. - Sarthaks eConnect | Largest Online Education Community
![standard enthalpy of vapourisation A HO) water 100°C is 40.66 kJmol.The internal energy of vapourisation of water 100°C (in kJmot) is [AIPMT (Prelims)-2012] (2) +40.66 (4) -43.76 funtor is 1 435 kcal/mol. (1) +43.76 (3) +37.56 standard enthalpy of vapourisation A HO) water 100°C is 40.66 kJmol.The internal energy of vapourisation of water 100°C (in kJmot) is [AIPMT (Prelims)-2012] (2) +40.66 (4) -43.76 funtor is 1 435 kcal/mol. (1) +43.76 (3) +37.56](https://toppr-doubts-media.s3.amazonaws.com/images/8783536/2a161f71-6f15-470c-af56-293d004f42a8.jpg)